How the EU-CIP project works
Our ambition is to build an EU-CiP Library of Contents that serves as basis for 11 national and regional CiPs. These portals will give patients, survivors, their families and caregivers access to trustworthy information and comprehensive, tailored, and up-to-date guidance.
The project builds on a highly interdisciplinary network of partners that cover all aspects ranging from the technological realisation of the portals, a best-practise approach to content generation and evaluation as well as ethical aspects.
Putting patients first, means involving them early on and throughout the whole project to ensure that their needs are met.
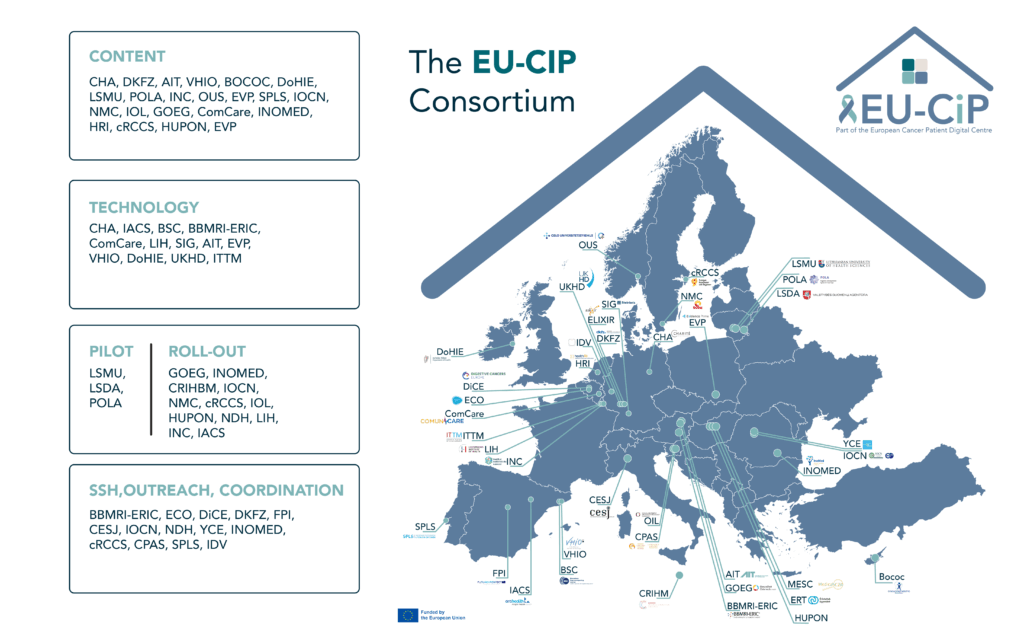
The EU-CIP partners and their roles
A total of 40 partners from 18 European countries work together in this project to build the European Cancer Information portals. This reflects the value and need of providing citizens with reliable information in a way that it is easily understandable. The EU-CIP partners bring expertise from areas like Cancer Research, Patient Engagement / Advocacy, Health Literacy, and many more. Explore the partner profiles.
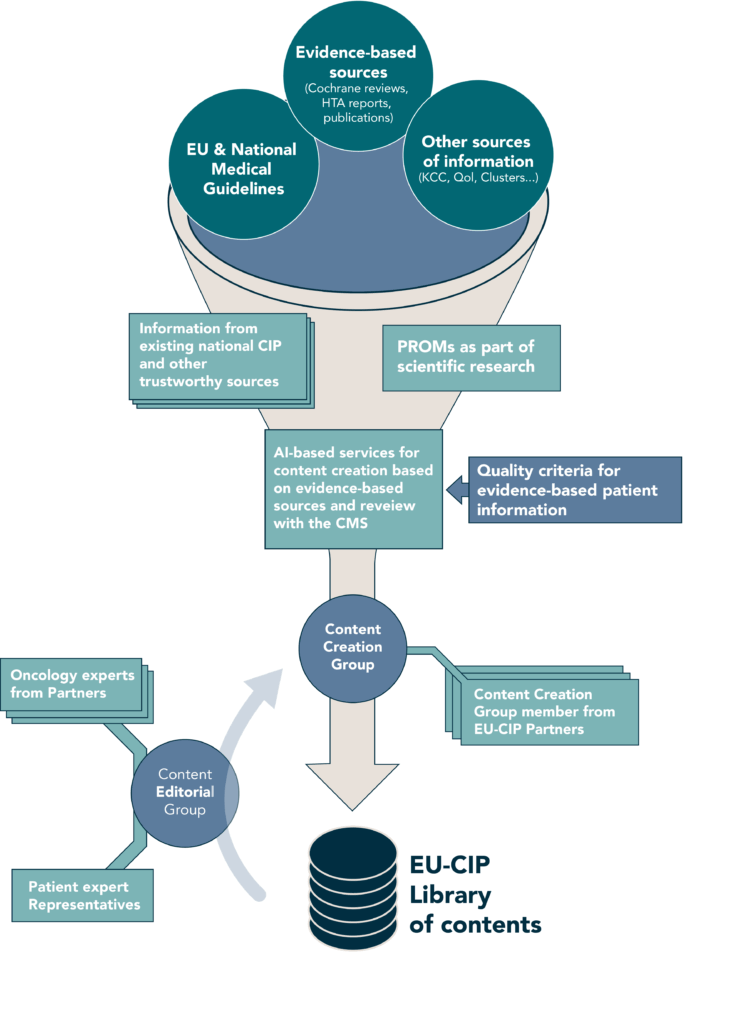
Content Creation and Editorial Workflow
The Content Creation Group will make use of existing information from various existing sources to create easy-to-understand, evidence-based information. These sources include participating Cancer Information Services (e.g., by the DKFZ), guidelines, the Knowledge Centre on Cancer (KCC).
The information will cover all stages of a patient’s journey from diagnosis, treatment options, rehabilitation, management of recurrence to palliative care. The Content Creation Group will use the AI-tools created in the project to provide input to the Content Editorial Board (see below) and will integrate its feedback after review.
The Content Editorial Board will uphold the quality, accuracy, and relevance of the presented information. The board is responsible for reviewing the content created by the Content Creation Group to ensure that it is correct and presented in a clear, concise, and easily understandable manner tailored to the specific needs of cancer patients, survivors, and caregivers.
Technology
The EU-CiP technical infrastructure will serve as the foundation of the ECPDC (see below). It will lay the groundwork for a scalable, flexible, and interoperable information portal that can serve the diverse needs of reliable, evidence-based information in lay-term language across Europe.
A comprehensive technical architecture for the EU-CIP will include a common Library of Content. This library will contain guideline-based information to be used and iterated by the Member States to match the information provided with their national and regional health care systems. Content generation is facilitated and managed by a suited Content Management System. This way, efforts by Member States and their participating partners can be reduced due to synergies in a co-creation process for the content.
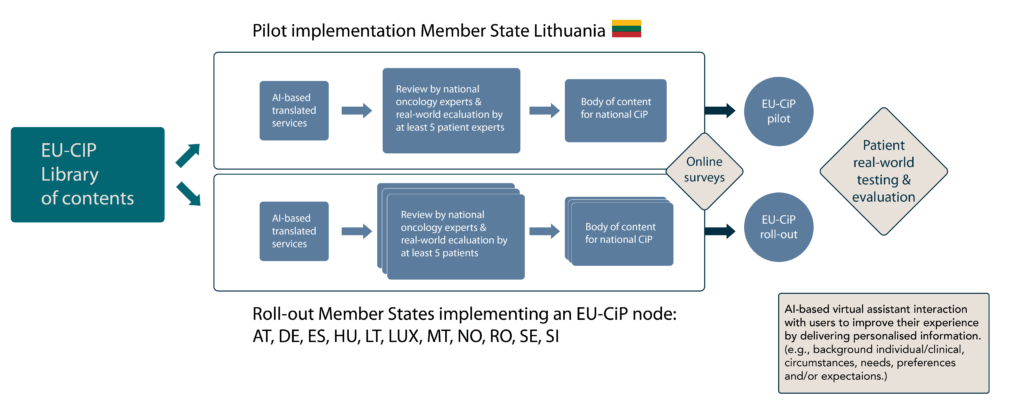
Pilot and Roll-out
The EU-CiP pilot will be conducted in Lithuania where the first Cancer Information Portal prototype will be established. It will consist of a pilot website which includes a good practice navigational structure and design to illustrate the content in the local language. The roll-out partner will benefit from lessons learned, good practice implementation solutions, tools and templates for their implementing solution. In a second step, a consortium of experts from multiple European countries will collaborate to implement portals in various national languages, ensuring broad accessibility and relevance across the Union. The design and implementation steps will be supervised by patient experts to ensure that the components and contents meet the requirements and needs of the patients.
Social Sciences and Humanities, Ethics, Outreach and Dissemination
Providing evidence-based information in easy-to-understand language needs tight integration of expertise from medical research, information technology development, health communication, social sciences, healthcare systems and patient advocacy..
Social sciences and humanities (SSH) play a central role in the EU-CIP project as they address challenges of health literacy promotion, patient empowerment, and information accessibility. This ensures that the portals meet the informational and psychosocial needs of cancer patients and their families with diverse backgrounds.
A separate work package coordinates the ethics requirements in the project, a crucial aspect as the portals will address people in challenging and vulnerable circumstances. As the users’ trust is crucial, processes need to be set up in a transparent and responsible manner.
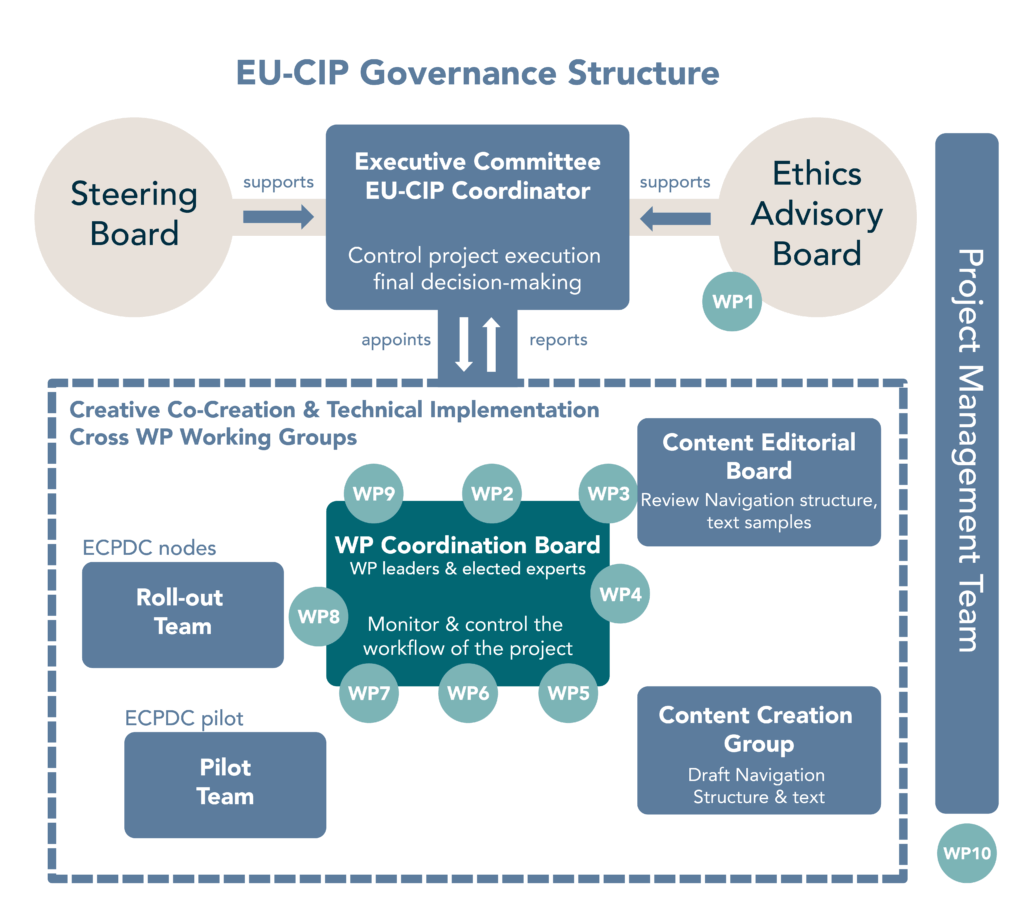
Governance structure
The project work is facilitated by a steering board and an ethics advisory board to include external expertise and guidance.
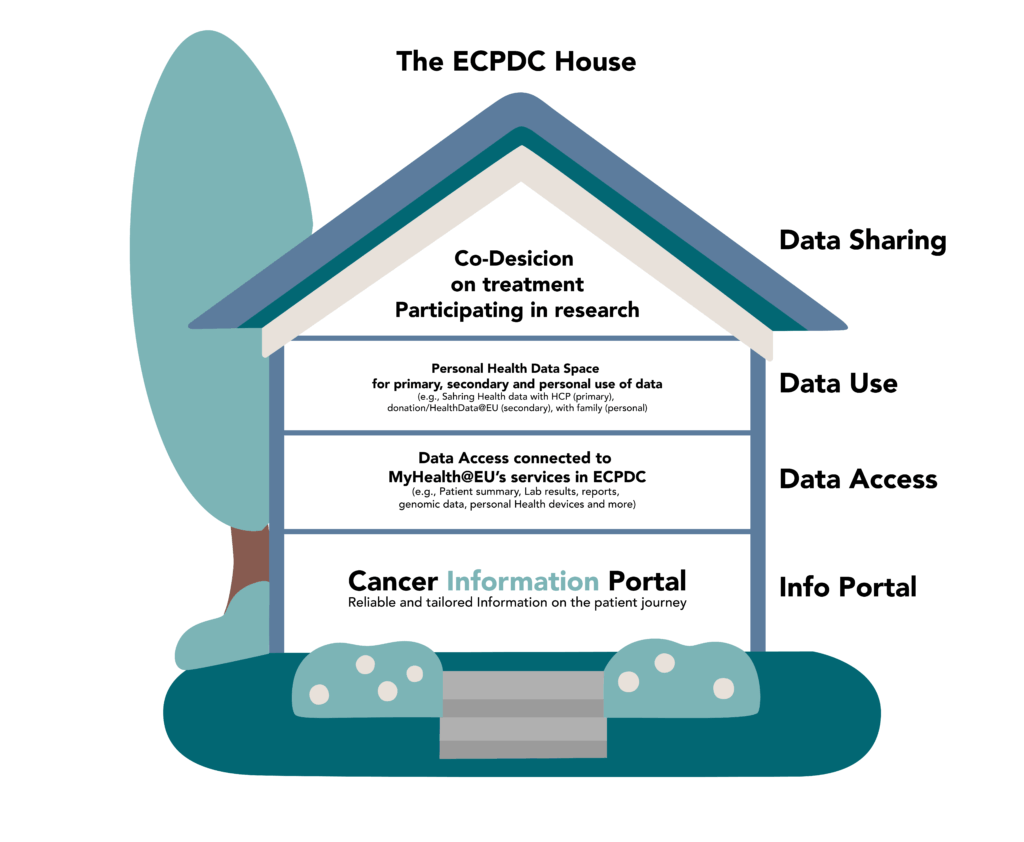
The bigger picture
The Cancer Info Portals are part of a larger vision to support citizens dealing with Cancer. ECPDC is short for “European Cancer Patient Digital Center” and the ECPDC house visualises this strategy as a building with multiple stories that represent different services and levels of engagement for the patients. The Cancer Info Portals, built in the EU-CiP project are a fundamental first step.
Working toward a healthy future
As one of the biggest medical challenges of our times, many efforts are focused on understanding cancer with all its variations and find the best possible options for treatment and care. EU-CIP partners up with related EU-funded cancer projects like CANDLE or UNCAN connect to generate the most benefit for society together. Overall these projects are part of EU-wide long-term strategies. You can find more information here:

